i3- hybridization|I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, : Pilipinas I3- is an interesting and difficult molecule to deal with when it comes to chemical bonding. Although the molecular geometry is linear as discussed earlier, the electronic geometry is trigonal bipyramidal. The structure of triiodide ions is such that the middle . Tingnan ang higit pa Some tools can help you in counter picking strategy. Dota 2 counter picker is a notable example of such. It allows you to search for advantage percentages against a hero or team during the selection, which helps you in making better choices . Also, Reddit and other community forums have been helpful to discuss strategies, ask for .
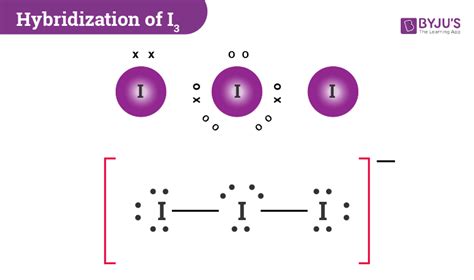
i3- hybridization,Learn how to draw the Lewis structure of I3- ion, a polyatomic molecule with a negative charge, and how to calculate its hybridization, molecular geometry, and polarity. Find out the properties and uses of I3- ion and its relation to starch. Tingnan ang higit paAnyone wanting to know in-depth about a molecule needs to learn about the Lewis Structure. Why is it so necessary to have an idea on . Tingnan ang higit paThe hybridization of I3 (Triiodide ion) is sp3d. The way we draw the structure of molecular compounds on paper is just a two-dimensional form. The understanding of . Tingnan ang higit paI3- is an interesting and difficult molecule to deal with when it comes to chemical bonding. Although the molecular geometry is linear as discussed earlier, the electronic geometry is trigonal bipyramidal. The structure of triiodide ions is such that the middle . Tingnan ang higit paIf we take another look at the Lewis Structure of the ion I3-, we can easily see that the central iodine has three lone pairs. The lone . Tingnan ang higit paHul 12, 2020 — This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the triiodide ion.

Nob 25, 2018 — Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. Find out the .Hun 7, 2022 — I3⁻ exhibits a linear geometry with bond angles of 180°, consistent with sp³d hybridization. The presence of the extra electron on the central iodine contributes to the ion’s .Learn how to calculate the hybridization of I3- ion, a linear anion formed by the bonding of I2 with I- ion. Find out the number of valence electrons, lone pairs, bond angles and molecular geometry of I3-.I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, I 3- is dsp 3 hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Elements in the first 2 periods of the .2 days ago — Learn how to determine the hybridization of I3-, a linear anion with sp3d hybridization, by using the formula or the lone pairs and valence electrons. See the Lewis .Okt 2, 2011 — I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.In the case of I3−, the iodine atoms utilize a type of Hybridization of I3− ion known as sp3d hybridization. In this section, we will discuss the hybridization of I3 and how to find the .Nob 26, 2020 — An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Triiodide Ion is also provided.
Iodine uses all the seven electrons present in 5s, 5p and 4d orbitals to form the hybrid orbitals. I3- is a linear ion formed by combining I2 molecule with me- ion in which I2 molecule acts as an acceptor and I- acts as an electron donor. The hybridization that I3- undergoes sp3 hybridization. The formula to calculate the hybridization of the .Okt 1, 2020 — #Hybridization of (I)3- , CHEMICAL BONDING#I3-,(I)3-#studyBibhaschemical bonding class 11,chemical bonding video lecture,hybridisation class 11 iit jee,how t.Abr 20, 2020 — About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .Okt 11, 2023 — The Lewis structure of triiodide [I 3] – consists of three identical iodine (I) atoms. One I atom acts as the central atom while the other two iodine atoms act as outer atoms. There are a total of 5 electron density regions around the central I atom in the I 3 – Lewis structure. Out of the 5 electron density regions, there are 2 bond pairs and 3 lone pairs of electrons on the .Mar 20, 2024 — This indicates that the hybridization of I3- is sp 3 d. Another way to determine the hybridization of I3- is by counting the number of valence electrons and lone pairs and adding them together. In this case, we have 3 lone pairs and 2 atoms donating valence electrons, giving us a total of 5, which also indicates sp 3 d hybridization. Key TakeawaysEne 30, 2023 — Prediction of sp 3 d, sp 3 d 2, and sp 3 d 3 Hybridization States. In case of sp 3 d, sp 3 d 2 and sp 3 d 3 hybridization state there is a common term sp 3 for which 4 sigma bonds are responsible. So, in addition to 4 sigma bonds, for each additional sigma, added one d orbital gradually as follows:-5σ bonds = 4σ bonds + 1 additional σ bond = sp 3 d hybridization. 6σ .i3- hybridizationHYBRIDIZATION OF I3. To know the crossbreeding of Triiodide particle, we will use a straightforward crossbreeding formula that is given as; Number of crossbreeding = electron + monovalent + (negative charge) – (positive charge)/2 If we glance at the iodine atoms, there are seven valence electrons in its outer shell, and 2 monovalent atoms are .Click here:point_up_2:to get an answer to your question :writing_hand:the hybridisation of central iodine atom in if5 i3 and i3 are respectivelysp Hybridization. This hybridization state is relatively uncommon. CO 2 and nitrile compounds (-CN, also called cyanide) are the most common. Acetylene (C 2 H 2) and N 2 are also sp hybridized.. Orbitals involved. Sp hybrid orbitals are composed of one s and one p orbital. Atoms with sp hybridization have two leftover unhybridized p orbitals which participate in pi bonding, .Ene 23, 2023 — Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that .Nob 26, 2020 — An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.Dis 16, 2021 — 1.6.3 Hybridization and VSEPR. Other than sp 3 hybridization, there are also other types of hybridization that include sp, sp 2, sp 3 d and sp 3 d 2.Usually the hybridization on a certain atom can simply be determined by .
Hul 12, 2023 — Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference of Be 2s .i3- hybridization I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, Ago 17, 2021 — Geometrical isomers. For some molecules in the Table, we note that there is more than one possible shape that would satisfy the VSEPR rules. For example, the XeF 2 molecule has a steric number of five and a trigonal bipyramidal geometry. There are three possible stereoisomers: one in which the F atoms occupy axial sites, resulting in linear molecule, one in .

May 18, 2014 — Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.13 hours ago — The Hybridization of IF5 is Sp3d2. The hybridization of a molecule can be understood in two ways:-The Theoretical part – The central atom Iodine atom has 7 valence electrons. Its electronic configuration in the ground state is – . Previous Article I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram.
Test Series. NEW. Talk to us
sp Hybridization. The beryllium atom in a gaseous BeCl 2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms. There are two regions of valence electron density in the BeCl 2 molecule that correspond to the two covalent Be–Cl bonds. To accommodate these two electron domains, two of the Be atom’s four valence .
i3- hybridization|I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity,
PH0 · Lewis Dot of Triiodide Ion I3
PH1 · Lewis Dot Structure of I3
PH2 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
PH3 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity,
PH4 · I3
PH5 · I3
PH6 · Hybridization of Iodine in Triiodide ion (I3)
PH7 · Hybridization of I3(
PH8 · Hybridization of I3 (
PH9 · Explain hybridization of I 3